REFERENCE: Department of Biology. 03rd March,2013.University of York, Wentworth Way, York,YO105DD,UK.http://www.york.ac.uk/biology/intranet/currentundergraduatestudents/common-pages/past-papers/. Accessed on 12th April,2013.
THE QUESTIONS BELOW WHERE THE ONES RELEVANT TO OUR COURSE
These question help me to review most of the topics covered in Biochemistry and it also guided me towards how much information I need to write to be awarded the assigned marks.
3. From the amino acid sequence shown below:
NLVETSGPCK
a) Choose the two amino acids with the most hydrophobic (non-polar) side-chains
and provide the names of the amino acids. (2 marks)
(i)
(ii)
b) Choose two amino acids that could participate in a salt-bridge interaction with
each other and state if the charge on each side-chain is positive or negative.
(3 marks)
(i)
(ii)
c) Choose an amino acid that could participate in a disulfide bond and name the
amino acid. (1 mark)
4. The figure below shows a region of a polypeptide chain.
a) Draw the resonance structure that leads to the partial double-bond character of
the peptide bond. (2 marks)
b) By describing the architecture of an alpha helix, explain why the resonance
structures, described in (a), lead to a helix having a net dipole moment.
(3 marks)
5. The diagram below shows an amino acid with a post-translational modification
(not all the atoms and bonds are shown).
a) Name the specific type of modification. (2 marks)
b) Provide the amino acid sequence context (sequence of three amino acids with
the first one being modified) in which it occurs. (2 marks)
___________________________________________________________
6. a) Draw two D-aldose pentose sugars in Fischer projection that are epimers.
(4 marks)
b) Circle the carbon centres whose stereochemistry is used to assign the chirality
of each aldose sugar molecule. (1 mark)
7. Draw docosahexaenoic acid (C22:6Δ4,7,10,13,16,19). (3 marks)
8. Where is a secreted protein synthesised? Describe the sequence of events that
must occur in order for such a protein to reach its destination. (4 marks)
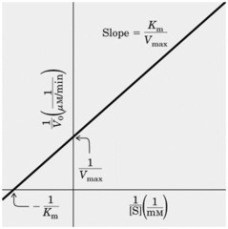
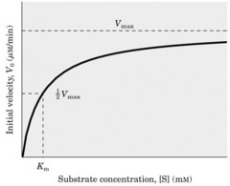
9. a) Draw a sketch graph to illustrate the relationship between substrate
concentration and rate of an enzyme-catalysed reaction, showing the
approximate values of the key enzymological parameters Vmax and KM. (4 marks)
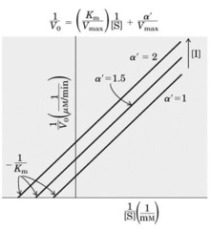
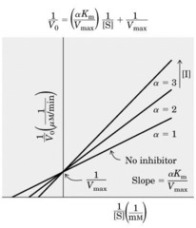
b) What happens to Vmax and KM in the presence of a non-competitive inhibitor?
(2 marks)
c) Illustrate how a non-competitive inhibitor interacts with an enzyme, and how
this is different from the interaction between a competitive inhibitor and the
enzyme. (3 marks)
10. a) State four features of a typical enzyme active site. (4 marks)
(i)
(ii)
(iii)
(iv)
b) The enzyme carbonic anhydrase catalyses the reversible interconversions of
carbon dioxide and water to carbonic acid (H2CO3). How does the active site
allow this reaction to be catalysed? (4 marks)
c) The conversion of carbon dioxide to carbonic acid occurs spontaneously at a
low rate. Why is it necessary for humans to have an enzyme to carry out this
process? (1 mark)
11. a) In the table below, rank the fuels glucose, fatty acids and glycogen for energy
density and preference. (3 marks)
Generating most (i) to least (iii)
amount of ATP per gram of fuel
Most (i) to least (iii) preferred by the
human body as an energy source
i)
ii)
iii)
b) The two rank orders do not agree. Why? (4 marks)
___________________________________________________________
12. a) Why is fermentation essential for mammals in the absence of oxygen?
(3 marks)
b) What is the role of the urea cycle? (2 marks)
c) Why is fructose-1,6-bisphosphate cut in half by aldolase? (2 marks)
___________________________________________________________
13. During extreme starvation when glycogen reserves have been fully depleted,
assume that the body’s energy needs are satisfied purely by the catabolism of
palmitate released from adipose tissue.
a) Given that the brain could consume up to 40% of the resting energy needs of
the body during extreme starvation, what percentage of the palmitate enters the
TCA cycle in non-brain tissues? (5 marks)
b) What percentage of the total CO2 is released by the brain? (1 mark)